Nucleus Research
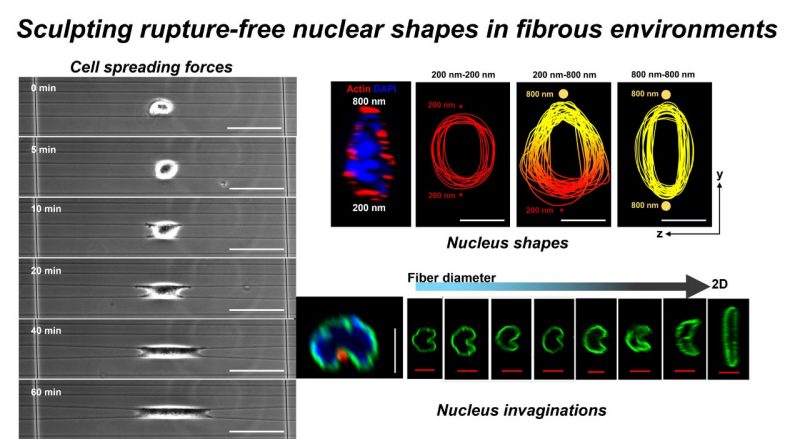
Cytoskeleton-mediated force transmission regulates nucleus morphology. How nuclei shaping occurs in fibrous in vivo environments remains poorly understood. Here a suspended nanofiber assay of precisely-tunable (nm-µm) diameters is used to quantify nucleus plasticity in fibrous environments mimicking the natural extracellular matrix. In contrast to the apical cap over the nucleus in cells on 2-dimensional surfaces, the cellular cytoskeleton of cells on fibers displays a uniform actin network caging the nucleus. The role of contractility-driven caging in sculpting nuclear shapes is investigated as cells spread on aligned single fibers, doublets, and multiple fibers of varying diameters. Cell contractility increases with fiber diameter due to increased focal adhesion clustering and density of actin stress fibers, which correlates with increased mechanosensitive transcription factor YAP translocation to the nucleus. Unexpectedly, large- and small-diameter fiber combinations lead to teardrop-shaped nuclei due to stress-fiber anisotropy across the cell. As cells spread on fibers, diameter-dependent invaginations that run the nucleus's length are formed at contact sites. The deepest and sharpest invaginations are insufficient to trigger nucleus rupture, often observed in 2D or confined systems. Invagination sites have higher enrichment of heterochromatin clusters indicative of deformation-induced epigenetic alterations. Overall, we describe the unknown adaptability of nuclei to fibrous environments and resultant sculpting of the nucleus shapes, with pathophysiological implications.
Collaboration with Prof. Kostas Konstantopoulos


