Cell Protrusions on Suspended Nanofiber Networks
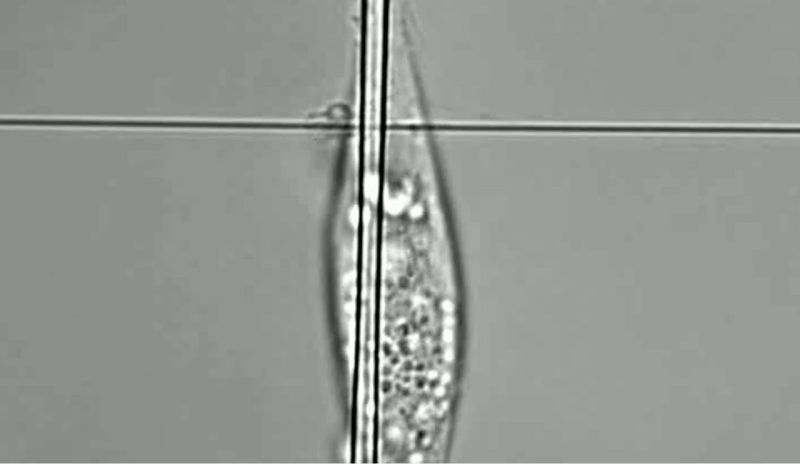
Protrusions are cytoplasmic extensions from the primary cell body that are used by cells to sense their fibrous surroundings. The ability of cells to extend protrusions underpins key biological processes such as cell migration and extracellular matrix (ECM) degradation. The importance of protrusions as the precursor to focal adhesion formation and subsequent migration in both normal and cancer cells has been consistently highlighted in the literature. Regarding ECM degradation, protrusions have been implicated in cleaving and reorganizing the surrounding ECM through the recruitment of matrix metalloproteinases (MMPs). Thus, elucidating the behavior of single cell protrusions is crucial towards expanding our understanding of how cells sense and interact with their surroundings, ultimately contributing to critical phenomena such as cancer metastasis, wound healing, etc.
Our Approach
At STEP Lab, we design suspended fiber networks with precisely tunable architecture and fiber diameter to investigate how single cells (both cancer and non-cancer) sense and interact with fibers by extending protrusions. We deposit large diameter “base fibers” orthogonal to smaller diameter “protrusive fibers”. The resulting mismatch in fiber curvature constrains bulk cell body migration along the base fiber, allowing us to investigate individual protrusions extended along the protrusive fibers. Using these suspended fiber networks we have observed the following protrusive behaviors:
Representative Publications
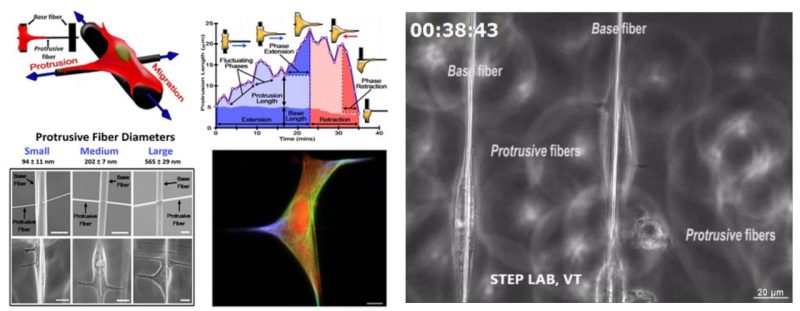
Cell migration is studied with the traditional focus on protrusion-driven cell body displacement, while less is known on morphodynamics of individual protrusions themselves, especially in fibrous environments mimicking extracellular matrix. Here, using suspended fibers, we report integrative and multiscale abilities to study protrusive behavior independent of cell body migration. By manipulating the diameter of fibers in orthogonal directions, we constrain cell migration along large diameter (2 μm) base fibers, while solely allowing cells to sense, initiate, and mature protrusions on orthogonally deposited high-curvature/low diameter (∼100, 200, and 600 nm) protrusive fibers and low-curvature (∼300 and 600 nm width) protrusive flat ribbons. In doing so, we report a set of morphodynamic metrics that precisely quantitate protrusion dynamics. Protrusion growth and maturation occur by rapid broadening at the base to achieve long lengths, a behavior dramatically influenced by curvature. While flat ribbons universally induce the formation of broad and long protrusions, we quantitatively protrutype protrusive behavior of two highly invasive cancer cell lines and find breast adenocarcinoma (MDA-MB-231) to exhibit sensitivity to fiber curvature higher than that of brain glioblastoma DBTRG05MG. Furthermore, while actin and microtubules localize within protrusions of all sizes, we quantify protrusion size-driven localization of vimentin and, contrary to current understanding, report that vimentin is not required to form protrusions. Using multiple protrusive fibers, we quantify high coordination between hierarchical branches of individual protrusions and describe how the spatial configuration of multiple protrusions regulates cell migratory state. Finally, we describe protrusion-driven shedding and collection of cytoplasmic debris.
Brian Koons, Paja Sharma, Zhou Ye, Apratim Mukherjee
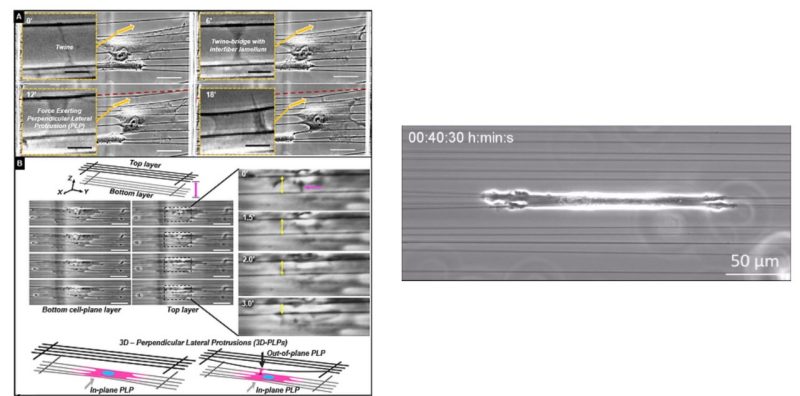
Aligned extracellular matrix fibers enable fibroblasts to undergo myofibroblastic activation and achieve elongated shapes. Activated fibroblasts adhere to the extracellular fibers and contract, perpetuating the alignment of these fibers. This poorly understood feedback process is critical in chronic fibrosis conditions, including cancer. Here, using fiber networks that serve as force sensors, we identify “3D perpendicular lateral protrusions” (3D-PLPs) that evolve from lateral cell extensions named twines. The specific morphology of PLPs enables them to exert force on parallel neighboring fibers, causing cells to increase their contractility. Twines originate from stratification of cyclic actin-waves traversing the cell and swing freely in 3D to engage neighboring fibers. Once engaged, a lamellum forms and extends multiple secondary twines, which fill in to form a sheet-like PLP, in a force- entailing process that transitions focal adhesions to elongated 3D-adhesions. Controlling the geometry of extracellular networks confirms that anisotropic fibrous environments support 3D-PLP formation and function, suggesting an explanation for cancer-associated desmoplastic expansion at single-cell resolution.
Abinash Padhi
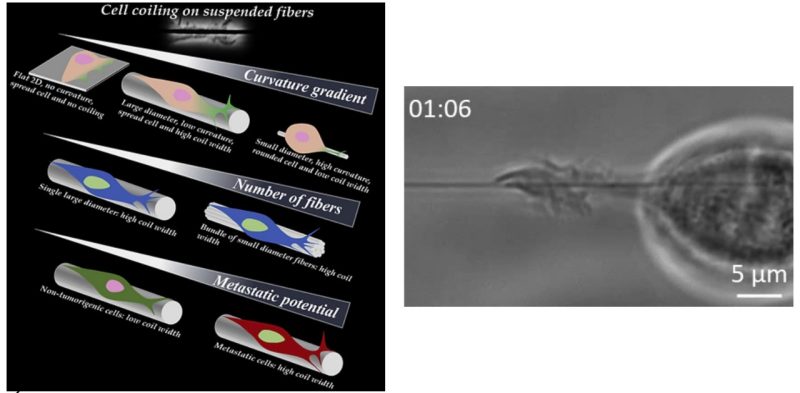
Metastatic cancer cells sense the complex and heterogeneous fibrous extracellular matrix (ECM) by formation of protrusions, and our knowledge of how cells physically recognize these fibers remains in its infancy. Here, using suspended ECM-mimicking isodiameter fibers ranging from 135 to 1,000 nm, we show that metastatic breast cancer cells sense fiber diameters differentially by coiling (wrapping-around) on them in a curvature-dependent manner, whereas non-tumorigenic cells exhibit diminished coiling. We report that coiling occurs at the tip of growing protrusions and the coil width and coiling rate increase in a curvature-dependent manner, but time to maximum coil width occurs biphasically. Interestingly, bundles of 135-nm diameter fibers recover coiling width and rate on 1,000-nm-diameter fibers. Coiling also coincides with curvature-dependent persistent and ballistic transport of endogenous granules inside the protrusions. Altogether, our results lay the groundwork to link bio-physical sensing with biological signaling to quantitate pro- and anti-invasive fibrous environments.
Apratim Mukherjee


