Cell Migration on Suspended Fibers
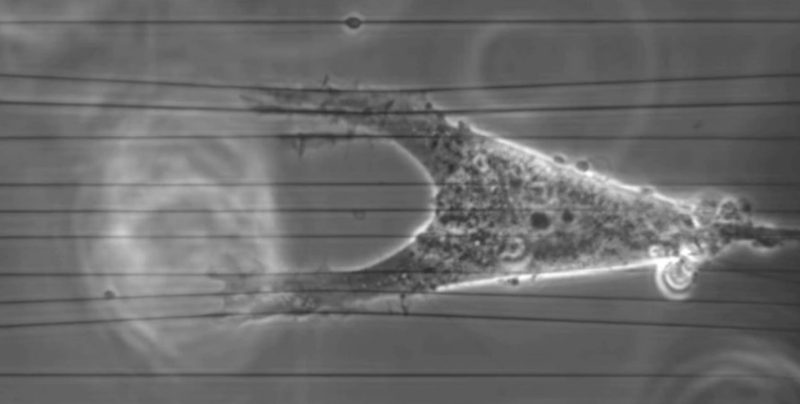
Cell migration is a fundamental process necessary for the creation of tissues during embryogenesis, immune surveillance, wound repair, inflammation, and the invasion and metastasis of cancer cells. All of these processes occur in the context of the extracellular matrix. For decades, there has been a concerted effort to understand the basic mechanisms of cell migration and other cellular behaviors, focused largely on cells cultured on 2D surfaces. Studies in vivo demonstrate that cells also use migratory approaches different than those observed on 2D surfaces in vitro, and even 2D migration in vivo occurs in the context of 3D tissue. Recently, there has been a move to apply the wealth of knowledge regarding what we know about 2D cell migration to understand cell migration within the context of 3D matrices and in vivo. Leaders in the field have identified several challenges to this task, which include the fact that migration in 3D matrices in vivo is quite different from 2D matrices, the difficulty creating relevant in vitro matrices that capture matrix composition and topography of in vivo microenvironments, and the challenges of manipulating the environment in vivo.
- Patti Keely (2015)
Our Approach
At STEP Lab, we deconstruct the effects of the various physical characteristics of the ECM on single and collective cell migration in precisely controlled environments. Through design of suspended fiber networks of varying attributes, we have demonstrated the following migratory responses:
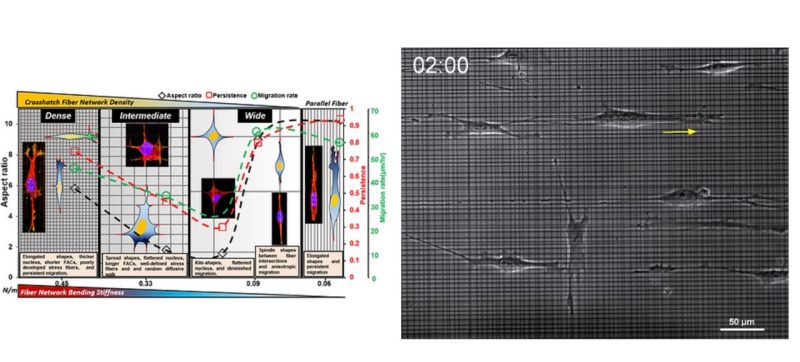
Biomechanical cues within tissue microenvironments are critical for maintaining homeostasis, and their disruption can contribute to malignant transformation and metastasis. Once transformed, metastatic cancer cells can migrate persistently by adapting(plasticity) to changes in the local fibrous extracellular matrix, and current strategies to recapitulate persistent migration rely exclusively on the use of aligned geometries. Here, the controlled interfiber spacing in suspended crosshatch networks of nanofibers induces cells to exhibit plasticity in migratory behavior (persistent and random) and the associated cytoskeletal arrangement. At dense spacing (3 and 6 mm), unexpectedly, elongated cells migrate persistently (in 1 dimension) at high speeds in 3-dimensional shapes with thick nuclei, and short focal adhesion cluster (FAC) lengths. With increased spacing (18 and 36 mm), cells attain 2-dimensional morphologies, have flattened nuclei and longer FACs, and migrate randomly by rapidly detaching their trailing edges that strain the nuclei by∼35%.At 54-mmspacing, kite-shaped cells become near stationary. Poorly developed filamentous actin stress fibers are found only in cells on 3-mm networks. Gene-expression profiling shows a decrease in transcriptional potential and a differential up-regulation of metabolic pathways. The consistency in observed phenotypes across cell lines supports using this platform to dissect hallmarks of plasticity in migration in vitro.
Aniket Jana
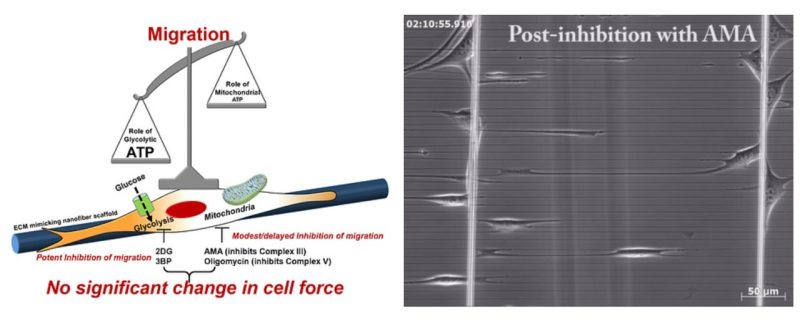
Cell migration is centrally involved in a myriad of physiological processes, including morphogenesis, wound healing, tissue repair, and metastatic growth. The bioenergetics that underlie migratory behavior are not fully understood, in part because of variations in cell culture media and utilization of experimental cell culture systems that do not model physiological connective extracellular fibrous networks. In this study, we evaluated the bioenergetics of C2C12 myoblast migration and force production on fibronectin-coated nanofiber scaffolds of controlled diameter and alignment, fabricated using a nonelectrospinning spinneret-based tunable engineered parameters (STEP) platform. The contribution of various metabolic pathways to cellular migration was determined using inhibitors of cellular respiration, ATP synthesis, glycolysis, or glucose uptake. Despite immediate effects on oxygen consumption, mitochondrial inhibition only modestly reduced cell migration velocity, whereas inhibitors of glycolysis and cellular glucose uptake led to striking decreases in migration. The migratory metabolic sensitivity was modifiable based on the substrates present in cell culture media. Cells cultured in galactose (instead of glucose) showed substantial migratory sensitivity to mitochondrial inhibition. We used nanonet force microscopy to determine the bioenergetic factors responsible for single-cell force production and observed that neither mitochondrial nor glycolytic inhibition altered single-cell force production. These data suggest that myoblast migration is heavily reliant on glycolysis in cells grown in conventional media. These studies have wide-ranging implications for the causes, consequences, and putative therapeutic treatments aimed at cellular migration.
- Abinash Padhi
- Selected as APS Select and nominated for best graduate student paper award 2019
- Study led by Brown Lab, Virginia Tech
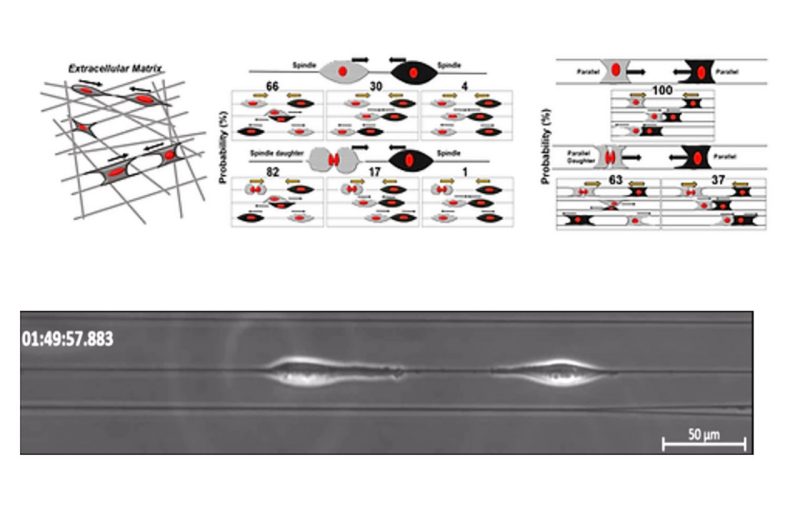
Contact inhibition of locomotion (CIL), in which cells repolarize and move away from contact, is now established as a fundamental driving force in development, repair, and disease biology. Much of what we know of CIL stems from studies on 2D substrates that fail to provide an essential biophysical cue – the curvature of extracellular matrix fibers. We discover rules controlling outcomes of cell-cell collisions on suspended nanofibers, and show them to be profoundly different from the stereotyped CIL behavior known on 2D substrates. Two approaching cells attached to a single fiber do not repolarize upon contact but rather usually migrate past one another. Fiber geometry modulates this behavior: when cells are attached to two fibers, reducing their freedom to reorient, only one of a pair of colliding cells repolarizes on contact, leading to the cell pair migrating as a single unit. CIL outcomes also change when one cell has recently divided and moves with high speed– cells more frequently walk past each other. In collisions with division in the two-fiber geometry, we also capture rare events where a daughter cell pushes the non-dividing cell along the fibers. In head-tail collisions, the slower leading cell always gain speed post contact. Our computational model of CIL in fiber geometries reproduces the core qualitative results of the experiments robustly to model parameters. Our model shows that the increased speed of post-division cells may be sufficient to explain their increased walk-past rate. Our results suggest that characterizing cell-cell interactions on flat substrates, channels, or micropatterns is not sufficient to predict interactions in a matrix – the geometry of the fiber can generate entirely new behaviors.
Jugroop Singh
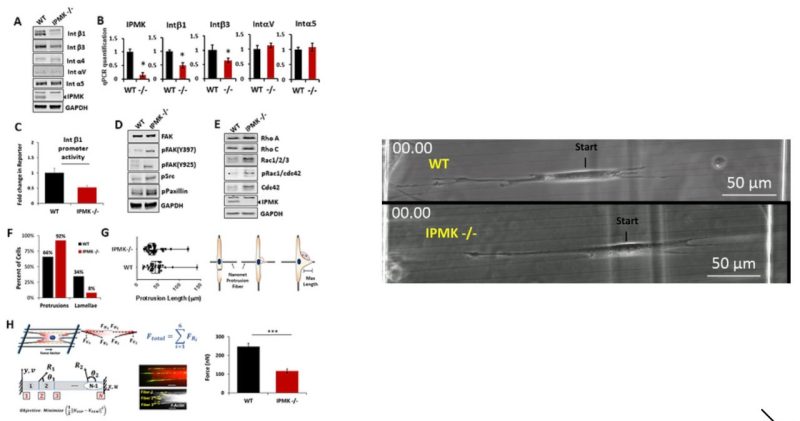
Metformin has been shown to alter cell adhesion protein expression, which is thought to play a role in its observed antitumor properties. We found that metformin treatment down‐regulated integrin β1 concomitant with the loss of inositol polyphosphate multikinase (IPMK) in murine myocytes, adipocytes, and hepatocytes. To determine if IPMK was upstream of integrin β1 expression, we examined IPMK‐/‐ mouse embryonic fibroblast cells and found that integrins β1and β3 gene expression was reduced by half, relative to wild‐type cells, whereas focal adhesion kinase (FAK) activity and Rho/Rac/Cdc42 protein levels were increased, resulting in migration defects. Using nanonet force microscopy, we determined that cell:extracellular matrix adhesion and cell contractility forces were decreased, confirming the functional relevance of integrin and Rho protein dysregulation. Pharmacological studies showed that inhibition of both FAK1 and proline‐rich tyrosine kinase 2 partially restored integrin β1 expression, suggesting negative regulation of integrin β1 by FAK. Together our data indicate that IPMK participates in the regulation of cell migration and provides a potential link between metformin and wound healing impairment.
Abinash Padhi, Study led by Kim Lab, Johns Hopkins University
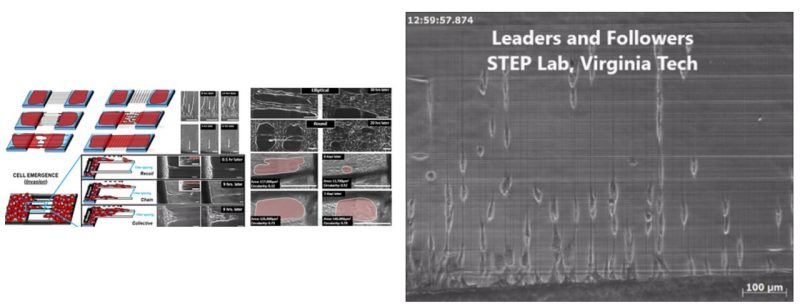
Cell emergence onto damaged or organized fibrous extracellular matrix (ECM) is a crucial precursor to collective cell migration in wound closure and cancer metastasis, respectively. However, there is a fundamental gap in our quantitative understanding of the role of local ECM size and arrangement in cell emergence based migration and local gap closure. Here, using ECM-mimicking nanofibers bridging cell monolayers, we describe a method to recapitulate and quantitatively describe these in vivo behaviors over multi spatial (single cell to cell sheets) and temporal (minutes-weeks) scales. On fiber arrays with large inter-fiber spacing, cells emerge either singularly by breaking cell-cell junctions analogous to release of a stretched rubber band (recoil) or in groups of few cells (chains), whereas on closely spaced fibers, multiple chains emerge collectively. Advancing cells on fibers form cell streams, which support suspended cell sheets (SCS) of varying sizes and curvatures. SCS converge to form local gaps that close based upon both the gap size and shape. We document cell stream spacing of 375 µm and larger hinders SCS advancement, thus providing abilities to engineer closing and non-closing gaps. Altogether, we highlight the importance of studying cell-fiber interactions and matrix structural remodeling in fundamental and translational cell biology.
Puja Sharma
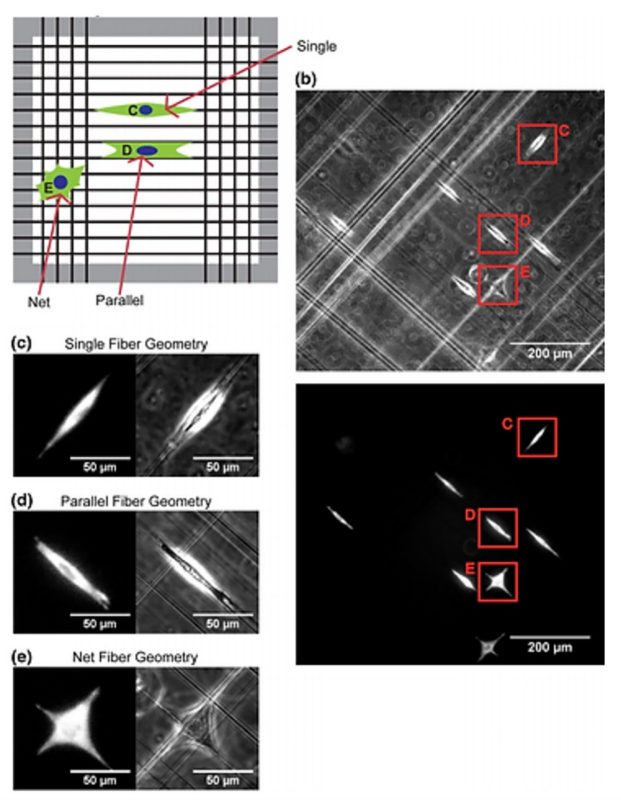
Understanding how cells migrate in fibrous environments is important in wound healing, immune function, and cancer progression. A key question is how fiber orientation and network geometry influence cell movement. Here we describe a quantitative, modeling-based approach toward identifying the mechanisms by which cells migrate in fibrous geometries having well controlled orientation. Specifically, U251 glioblastoma cells were seeded onto non-electrospinning Spinneret based tunable engineering parameters fiber substrates that consist of networks of suspended 400 nm diameter nanofibers. Cells were classified based on the local fiber geometry and cell migration dynamics observed by light microscopy. Cells were found in three distinct geometries: adhering two a single fiber, adhering to two parallel fibers, and adhering to a network of orthogonal fibers. Cells adhering to a single fiber or two parallel fibers can only move in one dimension along the fiber axis, whereas cells on a network of orthogonal fibers can move in two dimensions. We found that cells move faster and more persistently in 1D geometries than in 2D, with cell migration being faster on parallel fibers than on single fibers. To explain these behaviors mechanistically, we simulated cell migration in the three different geometries using a motor-clutch based model for cell traction forces. Using nearly identical parameter sets for each of the three cases, we found that the simulated cells naturally replicated the reduced migration in 2D relative to 1D geometries. In addition, the modestly faster 1D migration on parallel fibers relative to single fibers was captured using a correspondingly modest increase in the number of clutches to reflect increased surface area of adhesion on parallel fibers. Overall, the integrated modeling and experimental analysis shows that cell migration in response to varying fibrous geometries can be explained by a simple mechanical readout of geometry via a motor-clutch mechanism.
Aniket Jana, study led by Odde Lab, University of Minnesota
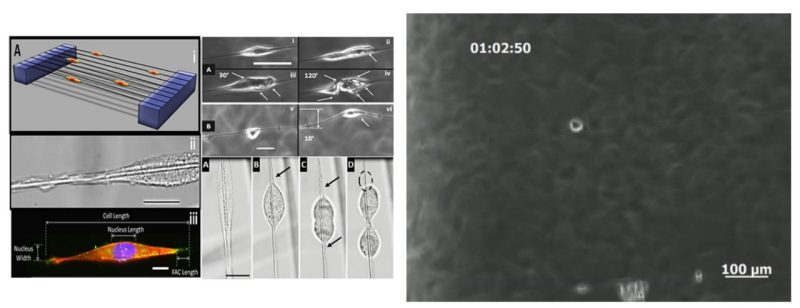
Cellular migration, persistence and associated cytoskeletal arrangement have been shown to be highly dependent on substrate stiffness (modulus: N/m2 and independent of geometry), but little is known on how cells respond to subtle changes in local geometry and structural stiffness (N/m). Here, using fibers of varying diameters (400, 700, and 1200 nm) and lengths (1mm and 2mm) deposited over hollow substrates, we demonstrate single mouse C2C12 cells attached to single suspended fibers form spindle morphologies sensitive to fiber mechanical properties. Over a wide range of increasing structural stiffness (2-100+ mN/m), cells exhibited decreased migration speeds ~57% (58-25 µm/hr) and average nucleus shape indices (NSI) ~26% (0.78 to 0.58), while the average paxillin focal adhesion cluster (FAC, formed at poles) length increased ~38% (8 to 11 µm). Furthermore, the increase in structural stiffness directly correlates with cellular persistence, with 60% of cells moving towards direction of increasing structural stiffness. At average structural stiffness (25±5 mN/m), cells put out longer focal adhesion cluster lengths on smaller diameters suggesting a conservation of FAC area and also exhibited higher NSI and migration speeds on larger diameter fibers. Interestingly, cells were observed to deform fibers locally or globally through forces applied through the FAC sites and cells undergoing mitosis were found to be attached to the FAC sites by single filamentous tethers. These varied reactions have implications in development biology and disease models as they describe a strong dependence of cellular behavior on the cell’s immediate mechanistic environment arising from alignment and geometry of fibers.
Sean Meehan
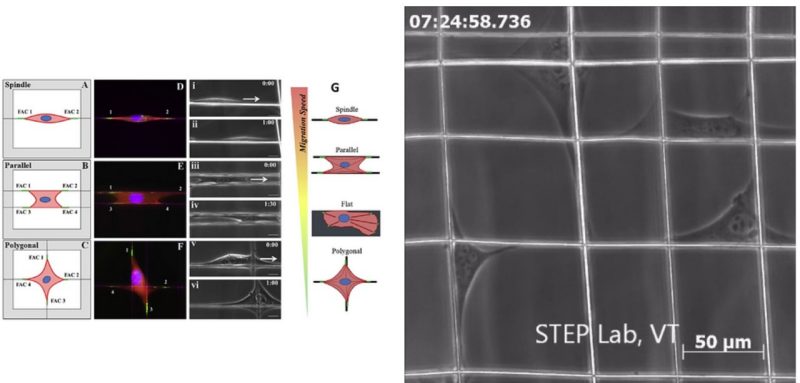
In the body, cells dynamically respond to chemical and mechanical cues from the extracellular matrix (ECM), yet precise mechanisms by which biophysical parameters (stiffness, topography and alignment) affect cell behavior remain unclear. Here, highly aligned and suspended multilayer polystyrene (PS) nanofiber scaffolds are used to study biophysical influences on focal adhesion complex (FAC) arrangement and associated migration behavior of mouse C2C12 cells arranged in specific shapes: spindle, parallel and polygonal. Furthermore, the role of cytoskeletal-altering drugs including blebbistatin, nocodazole and cytochalasin-D on FAC formation and migratory behavior is investigated. For the first time, this work reports that cells on suspended fiber networks, including cells with administered drugs, elongated along the fiber axes and developed longer (~ 4x) and more concentrated FAC clusters compared to cells on flat PS control substrates. Additionally, substrate designs which topographically restrict sites of cell attachment and align adhesions were found to promote higher migration speeds (spindle: 52 µm h-1, parallel: 39 µm h-1, polygonal: 25 µm h-1, flat: 32 µm h-1). This work demonstrates that suspended fiber topography-induced concentration of FACs along fiber axes generates increased migration potential as opposed to flat surfaces, which diffuse and randomly orient adhesions.
Kevin Sheets
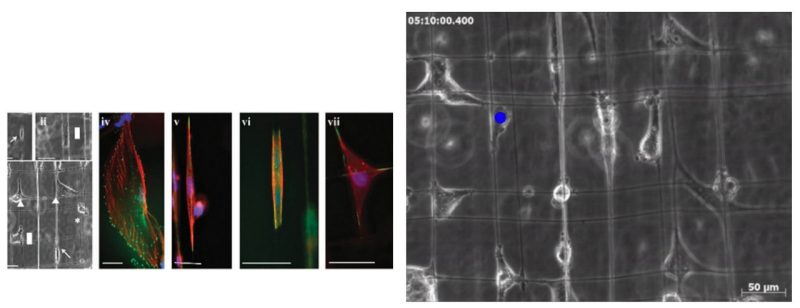
Investigating the mechanistic influence of the tumor microenvironment on cancer cell migration and membrane blebbing is crucial in the understanding and eventual arrest of cancer metastasis. In this study, we investigate the effect of suspended and aligned nanofibers on the glioma cytoskeleton, cell shape, migration and plasma membrane blebbing dynamics using a non-electrospinning fiber-manufacturing platform. Cells attached in repeatable shapes of spindle on single fibers, rectangular on two parallel fibers and polygonal on intersecting fibers. Structural stiffness (N m_1) of aligned and suspended nanofibers (average diameter: 400 nm, length: 4, 6, and 10 mm) was found to significantly alter the migration speed with higher migration on lower stiffness fibers. For cells attached to fibers and exhibiting blebbing, an increase in cellular spread area resulted in both reduced bleb count and bleb size with an overall increase in cell migration speed. Blebs no longer appeared past a critical cellular spread area of approximately 1400 mm2. Our results highlighting the influence of the mechanistic environment on the invasion dynamics of glioma cells add to the understanding of how biophysical components influence glioma cell migration and blebbing dynamics.
Puja Sharma


