Bio-Physical / Chemical Cues in Migration
Cells migrate through extracellular matrices, often through formation of integrin-mediated focal adhesions. This allows the cells to sense the external environment by exerting forces and thus allowing mechanotransduction. Measurement and calibration of these forces can provide insight in cell mechanobiology and pathology of diseases. To this effect, we have devised a fiber-based force measurement platform (Nanonet Force Microscopy, NFM) using ECM-mimicking anisotropic fiber arrangements and orthogonal large diameter fibers acting as supporting structures. The biological cues from the cytoskeletal arrangement of cells allows us to direct the force vectors along the dominant actin stress fibers at the poles of the cells, which are the regions of major focal adhesion clusters. A combination of beam mechanics and optimization allows us to extract forces exerted on the fibers by measuring the experimental deflections observed. We use NFM to understand the differences in healthy vs diseased cells, drug efficacies and role of forces in facilitating differentiation of stem cells into different lineages. NFM can be coupled with micromanipulators and cells can be subjected to Outside-In forces, thus allowing us to perform stress-strain tests at single cell level. This facilitates mechanical characterization of cells such as viscoelastic properties.
Our collaborative work led by Behkam Group
At STEP Lab, we design suspended fiber networks with precisely tunable fiber diameters to generate a meshwork of large (~2 μm) supporting fibers and orthogonal small diameter fibers (200, 500 or 800nm). Cells are aligned along the anisotropic arrangement of fibers which deflect under forces. Using the deflection measurements while modeling the fibers as beams with fixed boundary conditions and using the actin stress fiber information, we can calculate the forces exerted by single cells. We have employed this fiber based force platform to study the following cellular behaviors:
Representative Publications
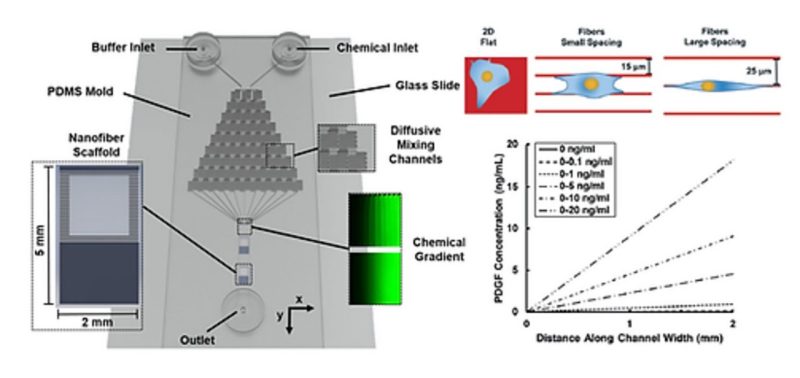
Persistent cell migration can occur due to anisotropy in the extracellular matrix (ECM), the gradient of a chemo-effector, or a combination of both. Through a variety of in vitro platforms, the contributions of either stimulus have been extensively studied, while the combined effect of both cues remains poorly described. Here, we report an integrative microfluidic chemotaxis assay device that enables the study of single cell chemotaxis on ECM-mimicking, aligned, and suspended nanofibers. Using this assay, we evaluated the effect of fiber spacing on the morphology and chemotaxis response of embryonic murine NIH/3T3 fibroblasts in the presence of temporally invariant, linear gradients of platelet-derived growth factor-BB (PDGF-BB). We found that the strength of PDGF-mediated chemotaxis response depends on not only the gradient slope but also the cell morphology. Low aspect ratio (3.4 ± 0.2) cells on flat substrata exhibited a chemotaxis response only at a PDGF-BB gradient of 0–10 ng mL−1. However, high aspect ratio (19.1 ± 0.7) spindle-shaped cells attached to individual fibers exhibited maximal chemotaxis response at a ten-fold shallower gradient of 0–1 ng mL− 1, which was robustly maintained up to 0–10 ng mL−1. Quadrilateral-shaped cells of intermediate aspect ratio (13.6 ± 0.8) attached to two fibers exhibited a weaker response compared to the spindle-shaped cells, but still stronger compared to cells attached to 2D featureless substrata. Through pharmacological inhibition, we show that the mesenchymal chemotaxis pathway is conserved in cells on fibers. Altogether, our findings show that chemotaxis on ECM-mimicking fibers is modulated by fiber spacing-driven cell shape and can be significantly different from the behavior observed on flat 2D substrata. We envisage that this microfluidic platform will have wide applicability in understanding the combined role of ECM architecture and chemotaxis in physiological and pathological processes.
Carmen Morrow


